Lupine Publishers | Journal of Chemical Sciences
Abstract
Introduction
The residual wastes (stubbles) are usually burnt in the field which leads to severe air pollution problems due to discharge of gaseous pollutants including CO, Ozone , N2O, NOx, SO2 , CH4, particulate matters ,smokes and smogs, hydrocarbons. Open burning of crop stubble also results in the emissions of harmful chemicals like polychlorinated dibenzo-p-dioxins, polycyclic aromatic hydrocarbons (PAH’s) and polychlorinated dibenzofurans (PCDFs) referred as as dioxins, besides loss of nutritional values of soil intermas of organic carbon, nitrogen, phosphorous and potassium in many states of India, especially Punjab, Haryana, Rajasthan and Uttar Pradesh. This is not that extent in other part of India. The main reasons are: larger length of the stubbles remains after harvesting in those states which cannot be economically covered under soil to enhance the fertility of the land and attempts to burn these long projected straws over the agricultural land. In the following paragraphs some alternatives to straw stubble burning are suggested [1].
Due to continuous depletion of non-renewable energy resources and high cost of chemicals due to import, at present there is a worldwide attention towards development of renewable resources of energy and chemicals for sustainable development for the welfare of mankind. For example: Economic production of bioethanol from lignocellusic biomass. Conversion of lignocellulosic plant materials to biochemicals is also regarded as one of the most promising alternatives to fossil fuels. Most abundantly available biomass in the countries like India and China are straws (rice, wheat, oats, rye, barleys, Zea Mays, corn stalks etc.), out of which rice straw occupies the first position and followed by wheat straw in terms of availability in Eastern, and North Eastern Indian states (West Bengal, Bihar, Assam, Orissa, Manipur etc.) whereas reverse is true for Northern India (U.P. Haryana, Punjab, Rajasthan etc.).
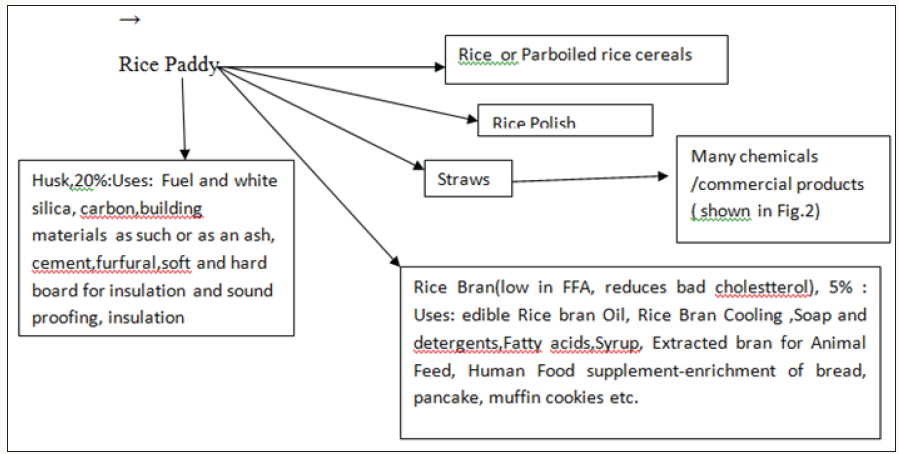
Conventional but Economic Uses of Rice Straw and Stubbles
b) Manuring/Composting with cowdung and others etc.
c) Briquettes
d) mats
e) Mushroom cultivation(as growth substrate)
f) Vegetables Cultivation
g) Animal Bedding material
h) Poultry Litter & Mulch
i) Feed for ruminants/Animal feed
j) Packaging goods for transporting goods &machineries
k) Frost prevention in horticulture
l) Strawberries (preventing damage to the fruit)
m) Thatching
n) Rope making
o) Traditional building materials, fibre boards,Particle board, insulation material
p) Energy (heat, power, fuels)
q) An intergrated solid state fermentation approach for production of enzymes from agro-wastes including straws
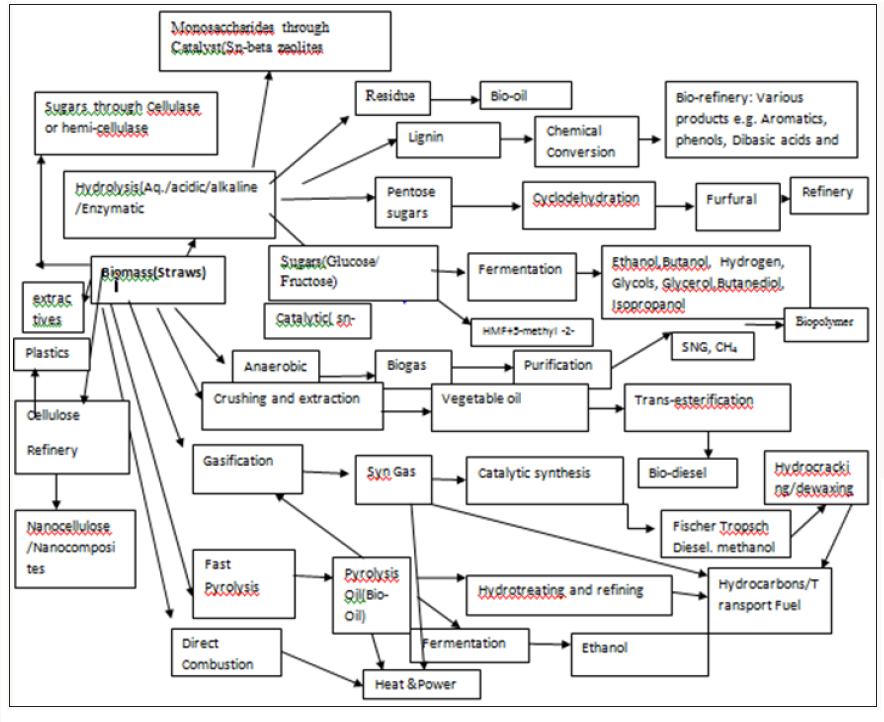
Lignocellulosic biomass could thus be utilized for both production of biofuels as well as biochemical’s due to its nature of renewability, low price, widespread availability and containing high content of pentose and hexose sugar polymers. These are detailed elsewhere [2-6]. Straw and Stubbles can be used for various Chemicals, valuable products and energy. The most notable products which can economically manufactured are: Pulp & Paper, Particle Board, Pulp and Paper Board, Straw board, board of rice husk.
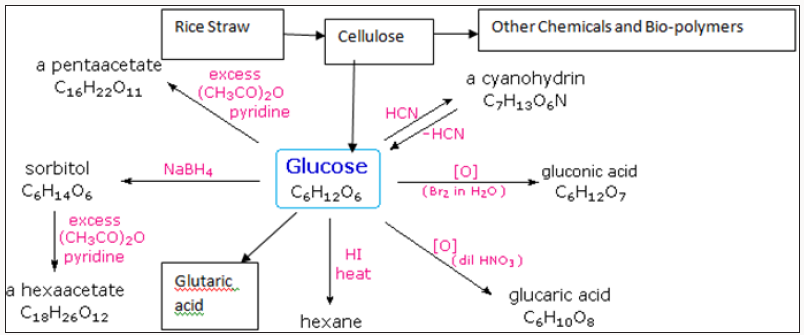
Energy technologies and thermal combustion consists of Non- Conventional uses of straws. Valuable chemicals include Cellulose, High Alpha cellulose, Plastics, Fuels and Energy,Bio-gas and in situ, Bio-oil, Nanocellulose and nano composites, Pentosans, Xylose, Xylitol, α-Cellulose, Glucose , Fructose, Hydroxy methyl Furan, Ethanol and host of many other chemicals [7-10]. These are shown in Figure 2.
Refineries based on Cellulose, Ethanol, Sucrose, Glucose, Lignin have been proposed and given elsewhere various Unit Operations and Processes involved to produce a biorefinery are as under:
a) Pulping
b) Gasification
c) Pyrolysis
d) Destructive distillation
e) Plasma Treatment
f) Chemical Treatment
g) Electron Irradiation
Chemical platform
a) Activated carbonb) Chemical transformation through Catalyst(Sn-beta zeolites)
c) Synthetic Fuel using Solar Furnace
d) Cellulose nano crystals and nanocomposites:
Cellulose nanocrystals have been largely applied as reinforcing fillers in the preparation of nano composites materials with improved mechanical and barrier properties.
Bio-chemical platform
Bio-chemical platforma) Renewable fuels: Ethanol, Biodiesel,Butanol, Hydrogen
b) Chemicals: Acetone,Furfurol,Propanediol, Ketones etc.
c) Organic Acids:Acetic, Lactic , Succinic, Gluconic, Butyric etc.
d) Bio-Energy: Lignate, Methane, Bio-gas, Heat, Electricity
e) Food & Feed: Single Cell Protein,Fat,Fiber, Sugar etc.
Chemistry of Formation
Monosachharides
D-Xylose,D-glucose, L-arabinose,Xylitol,fructose,D-mannose,DgalactoseHemicellulose
Furfural, through acid treatment, Biogas by anaerobic Digestion, concentrating to Animal Feed. Fructose /Fruit sugar →Hydroxymethyl furfural (HMF) →catalytic processes → Plastics, diesel fuel additives, or even diesel fuel [11,12].Chemical or Biochemical Platforms: Dilute acid hydrolysis of lignocelluloses:
Acids: Carboxylic acids such as formic acid, acetic acid, 3-hydroxy propionic acids, succinic acid, fumaric acids, Malic acids, Itaconic acids, Levulinic acid, Glucaric acids, glucuronic acid, Vanillic acids, Syringic acids, Ferulic acids, p-coumarlic acid. Amino acids like Aspartic acids,Glutamic acids, Aldehyde: SyringaldehydePolyphenols: glycerol, Arabitol, Xylitol, Sorbitol Lactones such as 3-hydroxy butyrolactone
Phenolics: p-hydroxy benzoic acids and vanillin However,aldopentose xylose (20-40% of the total carbohydrates are normally found in agricultural residues.
Reaction Schemes (4,9,21,29,30)
Chemical reaction consists of series, parallel and combination of series-parallel reactions
Cellulose (Glucan)→ Oligosaccharides →Glucose →HMF→Levulinic acid
Hemicellulose →Oligosachharides→Sugars( xylose,arabinose, glucose, mannose, galactose)
Pentoses ( Xylose/ Arabinose)→ Furfural→ Furfural resinification and condensation products
Hexoses(Glucose/ Fructose ) → HMF→ Levulinic acid+Formic acid→Succinic acid
Furfural and HMF
Chemistry of formation
Xylan Xylose Furfural: Hexosan →Hexose (Unit Cellulose) → 5-hydroxymethyl-2-furfural + 5-methyl -2-furfuralMechanism:
Hydrolysis:
xylan + water →H+Xylose
arbinan + water →H+arabinose
Dehydration:
Furan derivatives such as Furfural (2-furaldehyde), HMF (5-hydroxymethyl-2-furaldehyde), 2,5 furan dicarboxylic acids(33-35) are most important chemicals. HMF is produced industrially on a modest scale as a carbon-neutral feedstock for the production of fuels and other chemicals such as levulinic acid, gamma-valerolactone, or other byproducts. HMF itself has few applications and it is primarily produced in order to be converted into other more useful compounds [14-20]. Of these the most important is 2,5-furandicarboxylic acid, which has been proposed as a replacement for terephthalic acid in the production of polyesters. HMF can be converted to 2,5-dimethylfuran (DMF), a liquid that is a potential biofuel with a greater energy content than bioethanol. Hydrogenation gives 2,5-bis(hydroxymethyl) furan. Acid-catalysed hydrolysis converts HMF into gamma-valerolactone, with loss of formic acid. HMF is practically absent in fresh food, but it is naturally generated in sugar-containing food during heat-treatments like drying or cooking. Along with many other flavor- and color-related substances, HMF is formed in the Maillard reaction as well as during caramelization. In these foods it is also slowly generated during storage. Acid conditions favour generation of HMF. HMF is a well known component of baked goods. Upon toasting bread, the amount increases from 14.8 (5 min.) to 2024.8 mg/kg (60 min). It is a good wine storage time−temperature marker, especially in sweet wines such as Madeira and those sweetened with grape concentrate arrope [21-24].
Fermentation Technology
Bio-Ethanol
Sachharomyces cerevisiae, Zymomonous Mobilis, Clostidium thermocellum, Ruminococcus albus- a bacterium are generally used for conversion of cellulose to ethanol. Theoretically 1kg of sucrose on inversion, gives 1.053 kg of invert sugar, glucose and fructose combined together. Further,one tone of invert sugar yields 644.8 litres of absolute alcohol(ethanol of 100% ) or 678.7 litres of rectified spirit.The net CO2 emission of burning a biofuel like ethanol is zero since the cO2 emitted on combustion is equal to that aabsorbed from the atmosphere by photosynthesis during growth of the plant(sugarcane) used to manufacture ethanol.Inversion: C12H22O11+H2O→C6H12O6+C6H12O6
Sucrose + Water →Invertase→Glucose + Fructose (Invert Sugars)
Fermentation: C6H12O6→2C2H5OH+2CO2+27.8kCals xymase
Oxidation: C2H6O+3O2→2CO2+3H2O+Δ
Combined equation: C6H12O6+6O2→6CO2+6H2O+Δ
6CO2+6h2O+hv(light)→C6H12O6+6O2
Butanol
Biobutanol is produced by microbial fermentation, similar to bioethanol, and can be made from cellulosic feedstocks such as straws. The most commonly used microorganisms are strains of Clostridium acetobutylicum and Clostridium beijerinckii, C. Saccharoperbutylacetonicum and C. saccharobutylicum. In addition to butanol, these organisms also produce acetone and ethanol, so the process is often referred to as the “ABE (acetone-butanolethanol) fermentation [25-30]. Production of lactic acid from straw derived cellulose,cellulase production with Tricoderma citriviridae on solid bed, use of acid hydrolysates for lactic acid production using various strains such as Lactobacillus delbrueckii or lactobacillus pentosus can be explored.A number of products can be produced from sucrose as shown in Figure 4.Anaerobic Digestion
Biogas production (25,31-32), Anaerobic conversion of carbohydrate /cellulosics, especially of agricultural residues , has been considered for biogas ( methane) production which is typically, CH4=50-65%, CO2=35-50%, H2O=30-160g/m3, H2S=1.5-12.5g/ m3 inn presence of methane producing bacteria. Typically some of the methane forming microorganisms likes Methanaomonas, Methanococcous mazei n.sp. methannobacterium sohn genii n.sp. etc. are employed. The two best described pathways involve the use of acetic acid or inorganic carbon dioxide as terminal electron acceptors:CO2+4h2→CH4+2H2O
CH3COOH→CH4+2CO2
During anaerobic respiration of carbohydrates, H2 and acetate are formed in a ratio of 2:1 or lower, so H2 contributes only ca. 33% to methanogenesis [31], with acetate contributing the greater proportion. In some circumstances, for instance in the rumen, where acetate is largely absorbed into the bloodstream of the host, the contribution of H2 to methanogenesis is greater.
Buswell and Symons universal equation:
CnHaOb+(n-a/4-b/2)H2O→(n/2-a/8+b/4)CO2+(n/2+a/8-b/4)CH4
Thermo-chemical platform
Gasification TechnologyThe basic principles of Gasification technology are as under:
a. Steam Reforming of Straws:
Superheated steam reacts endothermally (consumes heat) with the carbonaceous components of straws to produce hydrogen and carbon monoxide fuel gases (synthesis gas or syngas)
b. Steam Reforming reaction: H2 O +C + Heat → H2 +CO
Water –gas shift reactions also occur simultaneously with the steam reforming reactions to yield additional hydrogen and carbon dioxide.
c. Water gas shift reaction: H2 O +CO→ H2- +CO2
Conclusion
For more Lupine Publishers Open Access Journals Please visit our website: http://www.lupinepublishers.com/
For more Journal of Chemical Sciences Journal articles Please Click Here: https://lupinepublishers.com/chemistry-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers https://lupinepublishers.us/
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers
Follow on Twitter : https://twitter.com/lupine_online

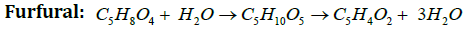
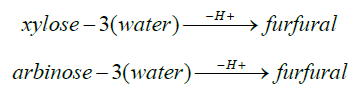


No comments:
Post a Comment