Thursday, 19 March 2020
Lupine Publishers: The Optimal Pain Management Methods Post Thoracic ...
Lupine Publishers: The Optimal Pain Management Methods Post Thoracic ...: Journal of Surgery | Lupine Publishers Abstract Post-operative pain control is one of the key factors that can aid in ...
Tuesday, 17 March 2020
Lupine Publishers: Lupine Publishers | The Prevention and Treatment o...
Lupine Publishers: Lupine Publishers | The Prevention and Treatment o...: Lupine Publishers | Open access Journal of Complimentary and Alternative Medicine Abstract Native people in West Timor In...
Monday, 16 March 2020
Lupine Publishers: Lupine Publishers | Acute Erythroblastic Leukemia ...
Lupine Publishers: Lupine Publishers | Acute Erythroblastic Leukemia ...: Lupine Publishers | Open Access Journal of Oncology and Medicine Abstract Acute erythroblastic leukemia is characteriz...
Friday, 13 March 2020
Lupine Publishers|Palauamine and Olympiadane Nano Molecules Incorporation into the Nano Polymeric Matrix (NPM) by Immersion of the Nano Polymeric Modified Electrode (NPME) as Molecular Enzymes and Drug Targets for Human Cancer Cells, Tissues and Tumors Treatment under Synchrotron and Synchrocyclotron Radiations
Lupine Publishers|chemistry journals
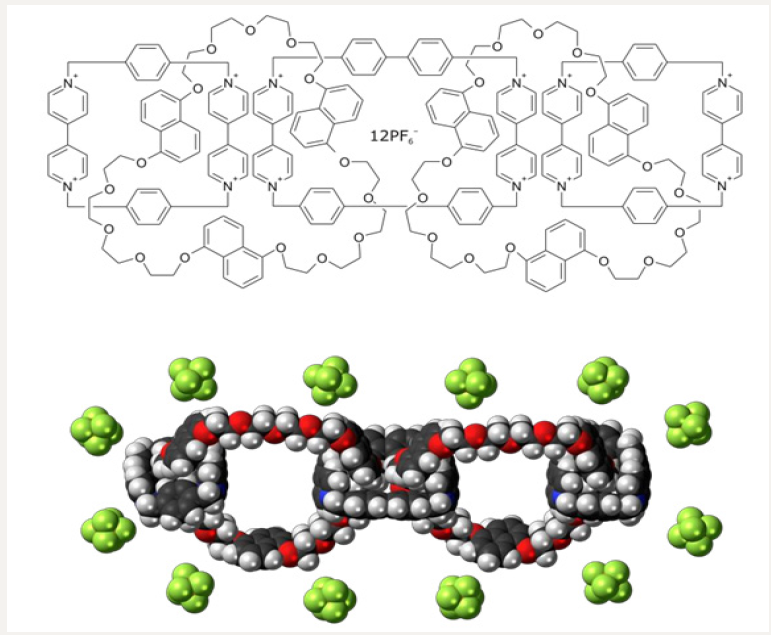
In the current editorial, we study Palau’amine and Olympiadane
Nano molecules (Figures 1 & 2) incorporation into the Nano
Polymeric Matrix (NPM) by immersion of the Nano Polymeric
Modified Electrode (NPME) as molecular enzymes and drug
targets for human cancer cells, tissues and tumors treatment
under synchrotron and synchrocyclotron radiations. In this regard,
the development of Chemical Modified Electrodes (CEMs) is at
present an area of great interest. CEMs can be divided broadly into
two main categories; namely, surface modified and bulk modified
electrodes. Methods of surface modification include adsorption,
covalent bonding, attachment of polymer Nano films, etc. Polymer
Nano film coated electrodes can be differentiated from other
modification methods such as adsorption and covalent bonding
in that they usually involve multilayer as opposed to monolayer
frequently encountered for the latter methods. The thicker Nano
films imply more active sites which lead to larger analytical
signals. This advantage coupled with other, their versatility and
wide applicability, makes polymer Nano film modified electrodes
particularly suitable for analytical applications [1–27].
Editorial
Figure 1: Molecular structure of Palau’amine Nano molecules.
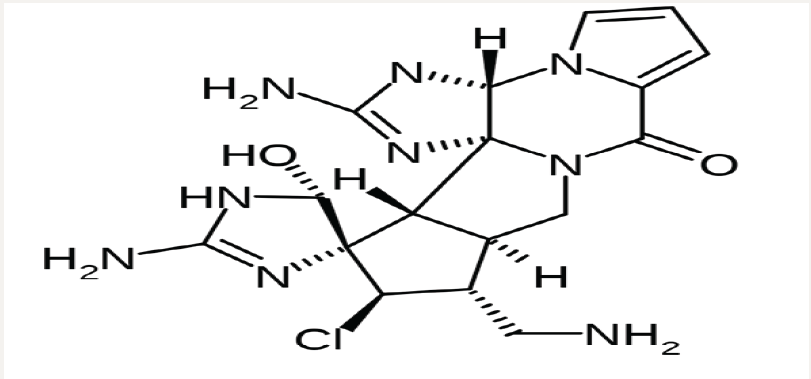

Figure 2: Molecular structure of Olympiadane Nano molecules.
Electrochemical polymerization offers the advantage of
reproducible deposition in terms of Nano film thickness and loading,
making the immobilization procedure of a metal–based electro
catalyst very simple and reliable for Palau’ amine and Olympiadane
Nano molecules–encapsulating Carbon nanotubes incorporation
into the Nano Polymeric Matrix (NPM) by immersion of the Nano
Polymeric Modified Electrode (NPME) as molecular enzymes and
drug targets for human cancer cells, tissues and tumors treatment
under synchrotron and synchrocyclotron radiations. Also, it must
be notice that the nature of working electrode substrate in electro
preparation of polymeric Nano film is very important, because
properties of polymeric Nano films depend on the working electrode
anti–cancer Nano materials. The ease and fast preparation and of
obtaining a new reproducible surface, the low residual current,
porous surface and low cost of Multi–Walled Carbon Nanotubes
(MWCNTs) paste are some advantages of Carbon Paste Electrode
(CPE) over all other solid electrodes [28–92].
On the other hand, it has been shown that, macrocyclic complexes of Palau’amine and Olympiadane Nano molecules– encapsulating Carbon nanotubes are interest as modifying agents because in basic media Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes redox centers show high catalytic activity towards the oxidation of small organic anti-cancer Nano compounds. The high–valence species of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes seem to act as strong oxidizing agents for low-electroactivity organic substrates. 1,2–Dioxetane (1,2– Dioxacyclobutane), 1,3–Dioxetane (1,3– Dioxacyclobutane), DMDM Hydantoin and Sulphobe as the anti–cancer organic intermediate products of methanol oxidation as well as formic acid, is important to investigate its electrochemical oxidation behavior in Palau’ amine and Olympiadane Nano molecules-encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations [93–110].
In this editorial, we decided to combine the above mentioned advantageous features for the aim of Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Furthermore, in this editorial, we prepared poly Nano films by electropolymerization at the surface of Multi-Walled Carbon Nanotubes (MWCNTs) paste electrode. Then, Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes were incorporated into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) in a solution. The modifier layer of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes at the electrode surface acts as a Nano catalyst for the treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations. Suitability of this Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes–modified polymeric Multi–Walled Carbon Nano tubes (MWCNTs) paste electrode toward the electrocatalytic treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations in alkaline medium at ambient temperature was investigated [111– 153].

On the other hand, it has been shown that, macrocyclic complexes of Palau’amine and Olympiadane Nano molecules– encapsulating Carbon nanotubes are interest as modifying agents because in basic media Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes redox centers show high catalytic activity towards the oxidation of small organic anti-cancer Nano compounds. The high–valence species of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes seem to act as strong oxidizing agents for low-electroactivity organic substrates. 1,2–Dioxetane (1,2– Dioxacyclobutane), 1,3–Dioxetane (1,3– Dioxacyclobutane), DMDM Hydantoin and Sulphobe as the anti–cancer organic intermediate products of methanol oxidation as well as formic acid, is important to investigate its electrochemical oxidation behavior in Palau’ amine and Olympiadane Nano molecules-encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations [93–110].
In this editorial, we decided to combine the above mentioned advantageous features for the aim of Palau’ amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes incorporation into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) as molecular enzymes and drug targets for human cancer cells, tissues and tumors treatment under synchrotron and synchrocyclotron radiations. Furthermore, in this editorial, we prepared poly Nano films by electropolymerization at the surface of Multi-Walled Carbon Nanotubes (MWCNTs) paste electrode. Then, Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes were incorporated into the Nano Polymeric Matrix (NPM) by immersion of the Nano Polymeric Modified Electrode (NPME) in a solution. The modifier layer of Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes at the electrode surface acts as a Nano catalyst for the treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations. Suitability of this Palau’amine and Olympiadane Nano molecules–encapsulating Carbon nanotubes–modified polymeric Multi–Walled Carbon Nano tubes (MWCNTs) paste electrode toward the electrocatalytic treatment of human cancer cells, tissues and tumors under synchrotron and synchrocyclotron radiations in alkaline medium at ambient temperature was investigated [111– 153].
For more Lupine
Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Open Access Journal on Chemistry articles Please Click Here:
https://lupinepublishers.com/chemistry-journal/
http://lupinepublishers.us/
For more Open Access Journal on Chemistry articles Please Click Here:
https://lupinepublishers.com/chemistry-journal/
Lupine Publishers: Lupine Publishers | Acute Erythroblastic Leukemia ...
Lupine Publishers: Lupine Publishers | Acute Erythroblastic Leukemia ...: Lupine Publishers | Open Access Journal of Oncology and Medicine Abstract Acute erythroblastic leukemia is characteriz...
Thursday, 12 March 2020
Lupine Publishers: Lupine Publishers | Principles of the Military Con...
Lupine Publishers: Lupine Publishers | Principles of the Military Con...: Lupine Publishers- Anthropological and Archaeological Sciences Journal Impact Factor Introduction A way from the regular th...
Lupine Publishers: Lupine Publishers | Principles of the Military Con...
Lupine Publishers: Lupine Publishers | Principles of the Military Con...: Lupine Publishers- Anthropological and Archaeological Sciences Journal Impact Factor Introduction A way from the regular th...
Wednesday, 11 March 2020
Lupine Publishers: Lupine Publishers | Principles of the Military Con...
Lupine Publishers: Lupine Publishers | Principles of the Military Con...: Lupine Publishers- Anthropological and Archaeological Sciences Journal Impact Factor Introduction A way from the regular th...
Tuesday, 10 March 2020
Lupine Publishers: Lupine Publishers | Historical Silahtaraga Power P...
Lupine Publishers: Lupine Publishers | Historical Silahtaraga Power P...: Lupine Publishers- Anthropological and Archaeological Sciences Journal Impact Factor Abstract In this article, which we prepare...
Friday, 6 March 2020
Lupine Publishers: Lupine Publishers | Historical Silahtaraga Power P...
Lupine Publishers: Lupine Publishers | Historical Silahtaraga Power P...: Lupine Publishers- Anthropological and Archaeological Sciences Journal Impact Factor Abstract In this article, which we prepare...
Thursday, 5 March 2020
Lupine publishers|An Efficient Protocol for the One Pot Synthesis of Pyranopyrazoles in Aqueous Medium using Triethanolamine as a Catalyst
Lupine publishers|Chemistry journal
Triethanolamine is an efficient and green catalyst for the synthesis of 6-amino-1, 4-dihydro-4-substituted-3-methylpyrano-[2,
3-c] pyrazole-5-carbonitrile in aqueous medium reflux conditions. The procedure is easier, eco friendly, simple with easy workup
affording good yield of the corresponding products.
Keywords: Multi component reaction, Water media, Pyranopyrazole, Catalyst, Triethanolamine
The present scenario for organic synthesis indicates the crave
for green and economical synthesis of organic compounds. One
of it is multi component synthesis. Strecker’s synthesis for amino
acids was the first report on multi component reaction [1]. Last
few decades show large development in it. The main aim of such
reactions is to fasten the reaction rate by reducing number of
steps involved and eventually increase the yield of reaction. In this
context to achieve great efficiency catalysts are employed. Catalysts
such as Nano α-Al2O3 supported ammonium dihydrogenphosphate
[2], tungstate sulfuric acid [3], Fe3-xTixO4@SO3H nanoparticles [4],
nano-titania sulfuric acid (15-nm TSA) [5], nanostructured MgO
[6], H14[NAP5W30O110] [7] and ZnO Nanoparticles [8].
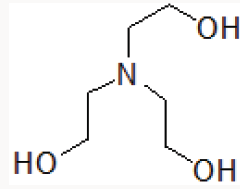
Organic catalysts such as Triethylamine [9] DABCO [10], Trishydroxymethyl aminomethane [11] are also reported in various organic transformations. Triethanolamine contains basic tertiary amine and primary alcoholic part (Figure 1).
It is used for activation of both CO2 and epoxides to convert them in to cyclic carbonates [12]. It is also reported as a legend for copper catalyzed hydroxylation of aryl halides in aqueous medium [13]. It is used as aqueous solvent for controllable preparation of ZnO nano flowers in sol gel technique [14]. Its aqueous solution is reported as electrolyte in CO2 Photo electro-conversion catalyzed by Cu- Doped Graphene-Titania Catalyst [15]. Also it is found to increase the rate of oxidation of mesitylene catalyzed by cobalt bromide [16]. It is used as sacrificial electron donor in photocatalytic system [17]. Furthermore; it improved the catalytic performance of CuBr/ PMDETA in the atom transfer radical polymerization [18]. It is also used as phase transfer catalyst for synthesis of 1-(arylsulfonyl) aryl/heterylmethanes [19]. It is used as medium for synthesis of 3-substituted coumarins using L-proline as a catalyst [20]. It is reported as catalyst in 10 mol% for synthesis of 2-amino-3-cyano- 4H-pyran derivatives under ultrasound irradiation at 600C [21].
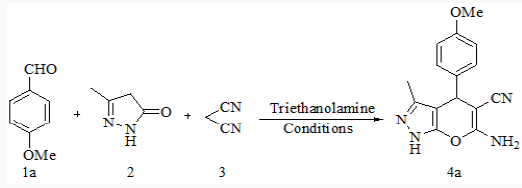
Synthesis of substituted pyrano-[2,3-d]-pyrimidines via one-pot three-component condensation of aromatic aldehydes, malononitrile and barbituric acid or 2-thiobarbituric acid using trace amounts of ionic liquid (choline chloride.ZnCl2) and triethanolamine (0.1Mol%) at 75°C with stirring and under ultrasound irradiation [22] is also reported in literature. Herein we successfully attempted a fast and simple protocol for the synthesis of 6-amino-1,4-dihydro-4-substituted-3-methylpyrano [2,3-c]- pyrazole-5-carbonitrile by the one pot three component reaction of aromatic aldehyde, malononitrile and 3-methyl-1H-pyrazol-5(4H)- one using triethanolamine as a catalyst [23].
To explore the synthetic application of triethanolamine, in the
present work we report the catalytic facet of it for the synthesis of
heterocyclic compounds bearing pyrazole skeleton. To optimize
the reaction conditions, we chose anisaldehyde as the prototype.
Initially, 10mol% of triethanolamine was taken for solvent free
reaction at room temperature. But the reaction afforded a low
yield of the product after 2 hour stirring. Then we used 10ml of
water for room temperature stirring [24]. After 2 hours stirring it
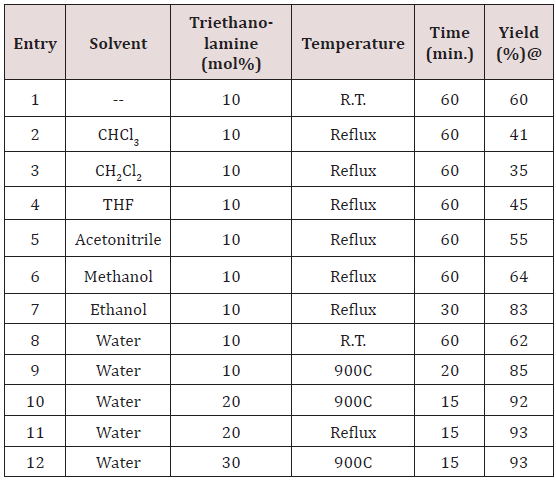
gave 62% of yield. The yield of reaction gets drastically changed on
increasing temperature. At 900C we got 85% of yield of the product.
When 20mol% of triethanolamine was used then we got 92% of
yield at 900C in 10 ml water. Other solvents were also studied
expecting better yield but other than ethanol and water we got
poor yields (Table 1). Further increase of temperature and amount
of triethanolamine did not improve yield significantly (Table 1).
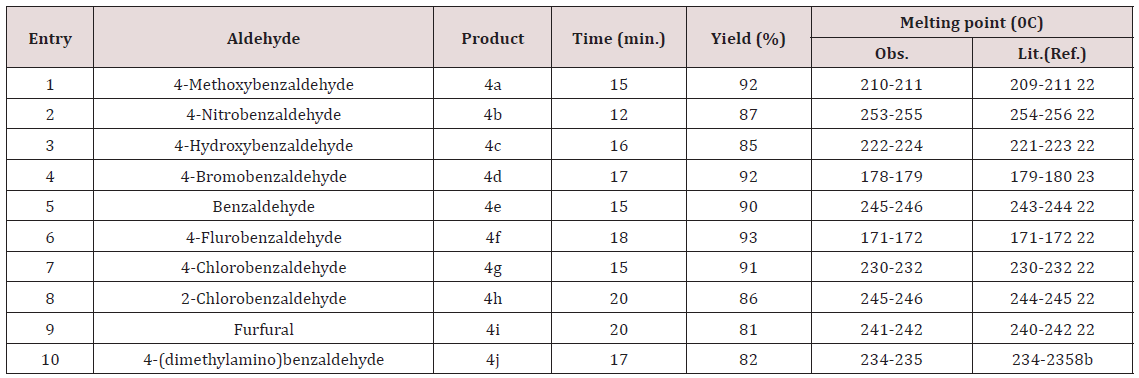
After optimizing the reaction conditions, differently substituted
aldehydes with electron donating as well as electron withdrawing
groups were reacted to examine the feasibility of this catalytic
reaction (Scheme 1).
Almost all aldehydes bearing various substituents such as –Cl, F, -NO2, -OMe etc afforded good yield of the corresponding products. All the synthesized compounds showed sharp peaks at 3410, 3356cm- 1(-NH2) and 2190cm-1(-CN) in IR spectra which supports for the formation of pyranopyrazole. The formed products being insoluble in water were easy to separate from the aqueous medium by simple filtration. The reason for catalytic activity of triethanolamine is it’s solubility in aqueous medium and basic nature. Products are simply purified by re crystallization with ethanol. Thus the protocol described herein is efficient for the synthesis of pyrazopyrazoles which do not need purification by column chromatography.
Model reaction* for anisaldehyde (2mmol), malononitrile (2mmol) and 3-methyl-1H-pyrazol-5(4H)-one (2mmol) using the above cited conditions @Isolated yield.
Melting points were recorded in open capillaries and were
uncorrected. Progress of reaction was monitored by TLC (30%
of ethyl acetate: n-hexane). IR spectra were taken by KBr disc on
Shimadzu IR Affinity 1 spectrophotometer [1]. H NMR spectra were
recorded on a Varian 400MHz spectrophotometer in the specified
solvents. Chemical shifts were expressed in 𝛿ppm relative to
TMS. Mass spectra were recorded on a Macro mass spectrometer
(Waters) by electro spray method (ES).
In summary, we have developed an efficient protocol for the
synthesis of pyranopyrazoles by a simple method using a catalytic
amount of triethanol amine. Herein; not only the yield of reaction is
improved but also the reaction time is reduced. The workup of the
reaction is very simple which make it easier to isolate the product.
The authors are thankful to The Principal, Vinayakrao Patil Mahavidyalaya, Vaijapur for providing laboratory facilities and The Director, SAIF, Chandigarh for NMR and Mass analysis.
Abstract
Keywords: Multi component reaction, Water media, Pyranopyrazole, Catalyst, Triethanolamine
Introduction
Organic catalysts such as Triethylamine [9] DABCO [10], Trishydroxymethyl aminomethane [11] are also reported in various organic transformations. Triethanolamine contains basic tertiary amine and primary alcoholic part (Figure 1).
It is used for activation of both CO2 and epoxides to convert them in to cyclic carbonates [12]. It is also reported as a legend for copper catalyzed hydroxylation of aryl halides in aqueous medium [13]. It is used as aqueous solvent for controllable preparation of ZnO nano flowers in sol gel technique [14]. Its aqueous solution is reported as electrolyte in CO2 Photo electro-conversion catalyzed by Cu- Doped Graphene-Titania Catalyst [15]. Also it is found to increase the rate of oxidation of mesitylene catalyzed by cobalt bromide [16]. It is used as sacrificial electron donor in photocatalytic system [17]. Furthermore; it improved the catalytic performance of CuBr/ PMDETA in the atom transfer radical polymerization [18]. It is also used as phase transfer catalyst for synthesis of 1-(arylsulfonyl) aryl/heterylmethanes [19]. It is used as medium for synthesis of 3-substituted coumarins using L-proline as a catalyst [20]. It is reported as catalyst in 10 mol% for synthesis of 2-amino-3-cyano- 4H-pyran derivatives under ultrasound irradiation at 600C [21].
Synthesis of substituted pyrano-[2,3-d]-pyrimidines via one-pot three-component condensation of aromatic aldehydes, malononitrile and barbituric acid or 2-thiobarbituric acid using trace amounts of ionic liquid (choline chloride.ZnCl2) and triethanolamine (0.1Mol%) at 75°C with stirring and under ultrasound irradiation [22] is also reported in literature. Herein we successfully attempted a fast and simple protocol for the synthesis of 6-amino-1,4-dihydro-4-substituted-3-methylpyrano [2,3-c]- pyrazole-5-carbonitrile by the one pot three component reaction of aromatic aldehyde, malononitrile and 3-methyl-1H-pyrazol-5(4H)- one using triethanolamine as a catalyst [23].
Results and Discussion
Almost all aldehydes bearing various substituents such as –Cl, F, -NO2, -OMe etc afforded good yield of the corresponding products. All the synthesized compounds showed sharp peaks at 3410, 3356cm- 1(-NH2) and 2190cm-1(-CN) in IR spectra which supports for the formation of pyranopyrazole. The formed products being insoluble in water were easy to separate from the aqueous medium by simple filtration. The reason for catalytic activity of triethanolamine is it’s solubility in aqueous medium and basic nature. Products are simply purified by re crystallization with ethanol. Thus the protocol described herein is efficient for the synthesis of pyrazopyrazoles which do not need purification by column chromatography.
Model reaction* for anisaldehyde (2mmol), malononitrile (2mmol) and 3-methyl-1H-pyrazol-5(4H)-one (2mmol) using the above cited conditions @Isolated yield.
Experimental
General method for the synthesis of 6-amino-1, 4-dihydro- 4-substituted-3-methylpyrano-[2,3-C]-pyrazole- 5-carbonitrile
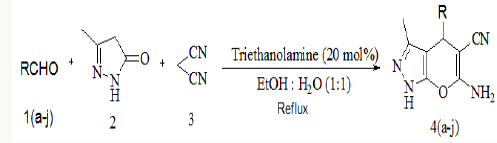
To a stirred mixture of aromatic aldehyde (2mmol), malononitrile (2mmol) and triethanolamine (20mol %) in 10ml of water, 3-methyl-1H-pyrazol-5(4H)-one (2mmol) was added. The resulting mixture was stirred and heated at 900C for appropriate reaction time (Table 2). After completion of reaction, the reaction mixture was cooled, filtered off the residue as the crude product which was further purified by re crystallization form ethanol (Scheme 2).
Scheme 2: General scheme for the one pot three component
synthesis of pyranopyazoles using triethanolamine
catalyst.

Representative Spectral Data
6-Amino-1,4-dihydro-4-(4-methoxyphenyl)-3-methylpyrano[ 2,3-c]pyrazole-5-carbonitrile (4a)
White solid, [1]H NMR (400 MHz, DMSO-d6): 𝛿 ppm 12.08 (s, 1H), 6.87-7.23 (m, 4H), 6.81 (bs, 2H), 4.45 (s, 1H), 3.78 (s, 3H), 1.81 (s, 3H); IR (KBr) cm-1: 3425, 3128, 2928, 2200, 1597, 1153, 1203; ES-MS m/z: 283.2 (M+1)+.6-Amino-2,4-dihydro-3-methyl-4-phenylpyrano[2,3-c] pyrazole- 5-carbonitrile (4e)
White solid, M.P. 245-246 0C; 1H NMR (400 MHz, DMSO-d6) : 𝛿 ppm 12.10 (s, 1H), 7.10-7.40 (m, 5H), 6.85 (s, bs, 2H), 4.60 (s, 1H), 1.78 (s, 3H); IR (KBr) cm−1 : 3410, 3356, 3167, 2990, 1646, 1596, 1399, 1276, 870; ES-MS m/z: 253 (M + 1) +.6 - A m i n o - 4 - ( 4 - c h l o r o p h e n y l ) - 3 - m e t h y l - 2 , 4 - dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4g)
Off-white solid, M.P. 230-2320C; 1H NMR (400 MHz, DMSO-d6): 𝛿 ppm 12.15 (s, 1H), 7.10–7.40 (m, 4H), 6.95 (s, bs, 2H), 4.63 (s, 1H), 1.80 (s, 3H); IR (KBr) cm−1 : 3478, 3035, 2985, 2193, 1647, 1596, 1398, 1284, 870; ES-MS m/z: 287 (M + 1) +.6-Amino-4-(4-N, N-dimethylaminophenyl)-3-methyl-2, 4-dihydropyrano[2, 3-c]pyrazole-5-carbonitrile (4j)
Yellow solid, M.P. 234-235 0C; 1H NMR (400 MHz, DMSO-d6): 𝛿 ppm 12.10 (s, 1H), 6.70-7.15 (m, 4H); 6.55 (s, bs, 2H), 4.40 (s, 1H); 2.85 (s, 6H), 1.78 (s, 3H); IR (KBr) cm−1 : 3385, 3172, 2957, 2189, 1644, 1601, 1397, 1279, 868; ES-MS m/z: 296 (M + 1) +.Conclusion
Acknowledgement
The authors are thankful to The Principal, Vinayakrao Patil Mahavidyalaya, Vaijapur for providing laboratory facilities and The Director, SAIF, Chandigarh for NMR and Mass analysis.
For more Lupine
Publishers Open Access Journals Please visit our website:
http://lupinepublishers.us/
For more Open Access Journal on Chemistry articles Please Click Here:
https://lupinepublishers.com/chemistry-journal/
http://lupinepublishers.us/
For more Open Access Journal on Chemistry articles Please Click Here:
https://lupinepublishers.com/chemistry-journal/
Lupine Publishers: Lupine Publishers | Peer Review of Statistics in S...
Lupine Publishers: Lupine Publishers | Peer Review of Statistics in S...: Lupine Publishers | Journal of Cardiology & Clinical Research Opinion Professor Peter Bacchetti’s excelle...
Wednesday, 4 March 2020
Lupine Publishers: Lupine Publishers | Subjection between Breast Canc...
Lupine Publishers: Lupine Publishers | Subjection between Breast Canc...: Lupine Publishers | Open Access Journal of Oncology and Medicine Abstract Increasing the effectiveness of antitumor the...
Tuesday, 3 March 2020
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction...
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction...: Lupine Publishers | Journal of Otolaryngology Research Impact Factor Abstract Objective: The pur...
Monday, 2 March 2020
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction...
Lupine Publishers: Lupine Publishers | Post Endodontic Pain Reduction...: Lupine Publishers | Journal of Otolaryngology Research Impact Factor Abstract Objective: The pur...
Subscribe to:
Comments (Atom)
Prediction of Physico-Chemical Properties for Polycyclic Aromatic Hydrocarbons Based on Electronic Characteristics of Molecules
Abstract QSPR models have been developed to predict of polycyclic aromatic hydrocarbons (PAHs) based on quantum chemical and integr...
-
Abstract QSPR models have been developed to predict of polycyclic aromatic hydrocarbons (PAHs) based on quantum chemical and integr...
-
Lupine Publishers | An archive of organic and inorganic chemical sciences Abstract The title compound TZ1 was synthesized by N-alkylation...
-
Si-Psa Tape with the Addition of Dolomite ( AOICS ) - Lupine Publishers It is well known that tapes on the silicone pressure-sensitive...